Two gene-based treatments for sickle cell disease recently approved by the Food and Drug Administration mark a major breakthrough in treatment of the condition that impacts more than 100,000 Americans.
Boston's Vertex Pharmaceuticals helped to create one of the therapies while Bluebird Bio in Somerville created the other.
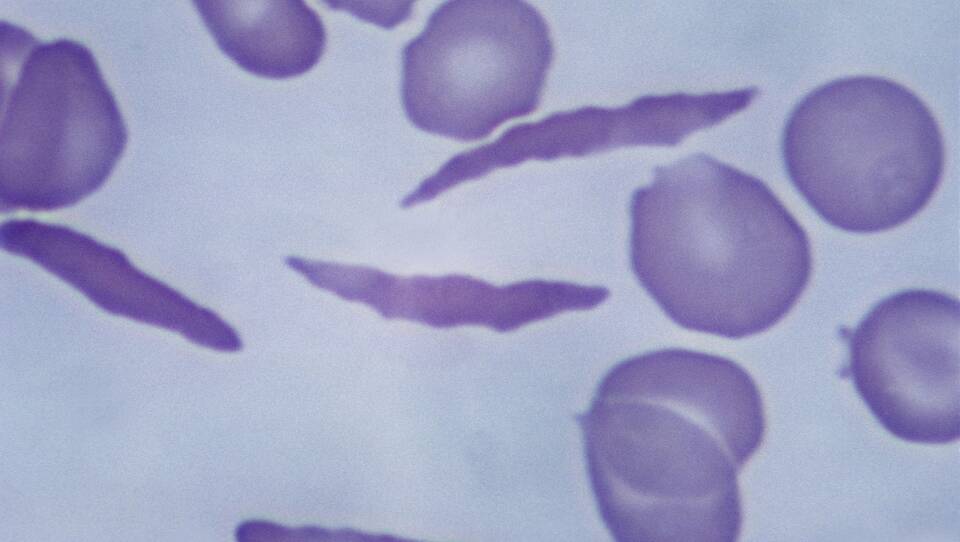
Sickle cell is a blood disorder that causes pain, infection, fatigue, breathing problems, dizziness and more.
Dr. Sharl Azar, medical director of the Massachusetts General Hospital Comprehensive Sickle Cell Disease Treatment Center, said on Greater Boston that while the treatment is not a total cure, it is a "transformative therapy."
Patients who get the treatment will still have sickle cell, but they will hopefully have far fewer complications from the disease, Azar said.
"We are very hopeful, and one of the things we are most excited about is that this has really kicked open the door in terms of placing society's attention on a disease that's been largely ignored," Azar said.
Carissa Juarez, a patient ambassador with the Massachusetts Sickle Cell Association who has a daughter with sickle cell, said news of the treatment is exciting, but the steep cost, the process and the potential side effects are overwhelming.
Azar said patients would need to provide stem cells, undergo a bone marrow transplant and do chemotherapy, all of which can take up to six months. The chemotherapy process would likely make a patient infertile.
Juarez said she and her daughter have talked about it extensively with excitement and apprehension.
"It's still great news because it's something. It's showing that something is being done. We are trying to get there," Juarez said.
Azar said companies are likely already working on future iterations of the therapy. He said the "dream therapy" would be gene editing in pill form.
Watch: Could a gene therapy drug cure Sickle Cell Disease?