Researchers are contacting volunteers who participated in the Moderna and Pfizer vaccine trials to tell them whether or not they received the vaccine or a placebo. If they did get a placebo, the volunteers are being offered the real vaccine.
The decision to “unblind” the vaccine trials is unusual. Participants in medical trials are rarely told what they received while the trial is ongoing. But as many as two-thirds of the volunteers in the Moderna study are in high-risk categories. Researchers told GBH News that with a vaccine that is 95 percent effective against a virus this dangerous, alerting participants is the ethical thing to do.
“The volunteers are heroes,” said Dr. Lindsey Baden of Brigham and Women’s Hospital, who’s one of the lead investigators in the Moderna study. “They are committing their time, and they’re participating so that we can learn how the vaccine behaves and prevents illness, that then the rest of us benefit from.”
Beyond any ethical obligation, Baden said, there’s a scientific reason for unblinding them — it allows researchers to continue gathering data from participants. If someone in the study goes and gets a shot on their own, just to be safe, their data becomes useless.
"The key is keeping people in the study,” Baden said. “If people leave the study, then we learn nothing."
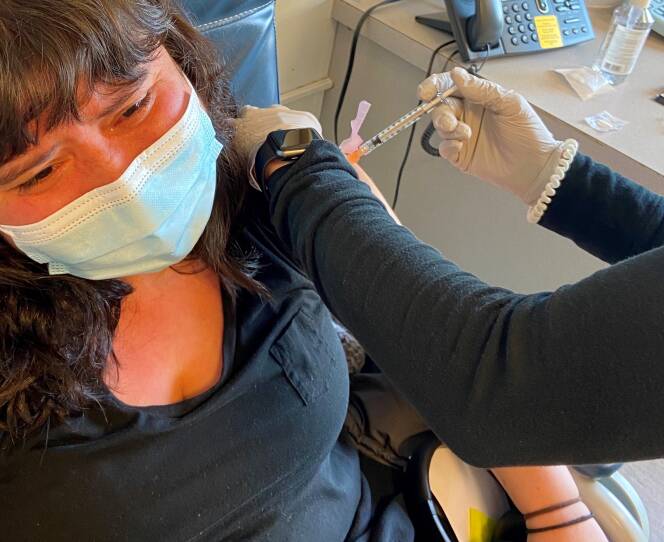
Geri Medina of Jamaica Plain participated in the Moderna trial over the summer, and she received the placebo. When Medina was given the actual vaccine, she was brought to tears with emotion. Medina said she was in awe at the science that made a vaccine available so quickly, overcome by the weight of all of those who died before one was available, and relieved at being that much closer to an end to the pandemic for all of us.
To hear the full radio story, click the “play” button above.




