At-home COVID-19 tests are now for sale at local pharmacies.
And while it may feel like quick, at-home testing is available a year too late, experts say if it catches on, this kind of testing could be a crucial tool to finally bringing an end to the pandemic.
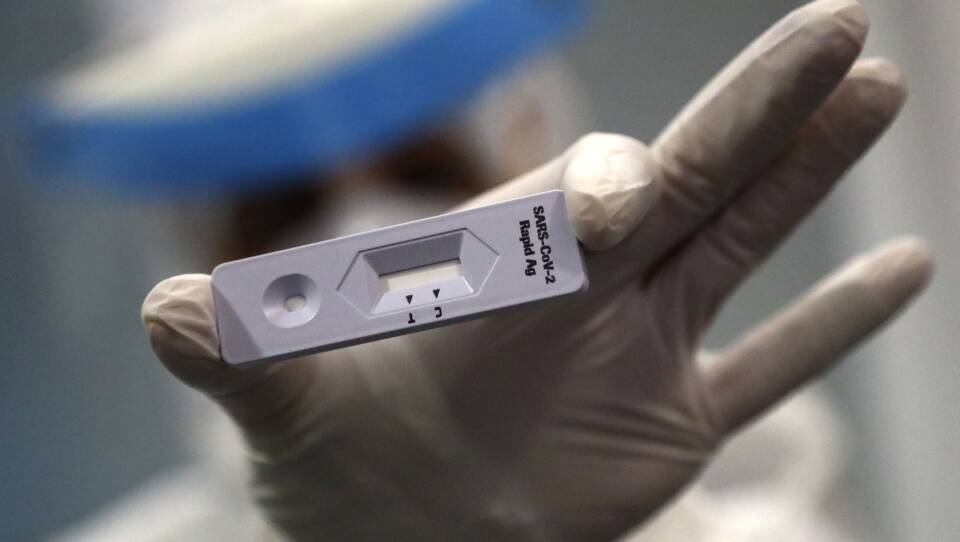
Rapid antigen tests, as they’re called, can quickly detect the presence of molecules found on the surface of the COVID-19 virus. At-home versions made by Abbott, Quidel and Ellume just recently hit the market . The tiny Cambridge-based start-up E25Bio has an application pending with the U.S. Food and Drug Administration and hopes to be joining those ranks soon.
Rapid antigen tests are cheaper to produce and easier to use than PCR tests, which have become the gold standard for detecting COVID-19 because they are the most accurate. But PCR tests must be analyzed in a laboratory that can extract genetic material from a sample to detect the presence of the virus. The results can sometimes come back days later.
More Local News
The FDA has moved slowly and deliberately as it has considered the quicker at-home antigen testing.
"Not getting these tests out to the country and not authorizing them in a timely fashion was potentially one of the most damaging decisions that we have made as a country, and one of the most important tools that we did not deploy — and really still have not deployed — in the type of way that would have prevented that kind of spread," said Michael Mina, an assistant professor of epidemiology at the Harvard T.H. Chan School of Public Health who’s been a vocal advocate for rapid testing.
The FDA was slow to approve rapid antigen tests out of a concern that they are less accurate than PCR tests, Mina said. A false negative could lead someone to accidentally expose others.
But last month, the FDA shifted course, approving at-home testing with a new caveat intended to bring accuracy levels up to the standard expected from PCR testing. They call it "serial testing," and it simply means that people should take the test a few times before they are confident of the result. That is why each box of the newly-approved at-home products come with at least two tests.
"The likelihood that a test would get a false negative twice in a row is much lower than the likelihood that you would get a false negative in one single test," said Zoe McLaren, a health economist and associate professor of public policy at the University of Maryland Baltimore County.
One exception is the Ellume rapid antigen test, which was able to show high enough accuracy rates to get FDA approval without the serial testing requirement. That test became available at CVS on April 19.
An FDA decision on at-home use of the E25Bio test could come at any time, and approval would be a big achievement for the tiny company.
E25Bio was launched by scientists from Harvard and MIT in 2018, and it has fewer than 10 full-time employees. Despite that, the company’s CEO, Prashant Chouta, said that once its test is approved, the company could immediately start producing a million tests a day and scale up to two million within a month, using contract manufacturing facilities around the globe.
The antigen tests typically consist of a swab, a small plastic vial filled with a liquid and a testing device with a paper strip, which is roughly the size of a stick of gum. Users swab the inside of each nostril, place the swab in the vial of liquid solution and squeeze a few drops onto the testing device. In 10 to15 minutes, it tells someone if they have COVID-19.
"It is very small, and it's very, very, very, simple," Chouta said, noting that his company’s version allows people to synch up with a smartphone app to share their results with healthcare providers or anyone else they wish to notify. “There are some complex reactions happening in that piece of paper when people put a sample in.”
More than a year into the pandemic, it's clear that earlier access to convenient and quick testing like this would have been invaluable for reducing the spread of the virus — especially before vaccines were available. But McLaren said it's not too late for them to be useful now, especially since some people can't be vaccinated.
"For example, none of our kids are vaccinated yet," she said. "We hope to have an authorized vaccine for them in the next few months, but we don't know when that's going to happen, and then we don't know how long it's going to take to ramp up vaccination among children."
Regular at-home testing could prevent outbreaks as schools open back up, McLaren said. They also could be effective as people begin attending large events again.
"You could imagine having the situation where to get into a packed concert hall, you either have to show that you had been fully vaccinated already or you agree to do a rapid antigen test, and you're only admitted if it was a negative test," she said. "That's a way that we could make sure that everyone had access to the concert hall while keeping everybody as safe as possible."
Also, McLaren said, while vaccines have been successful so far in reducing the spread of COVID-19, the virus is continuing to rapidly mutate into new variants, so it's important to have options available to quickly detect new infections.
A national public education campaign is needed to make it clear to people that these newly available tests are accurate and important, she said.
"The demand is much lower than it should be. Everybody should want one," McLaren said. "But there are people who are like, 'Yeah, I'm going to wait and see. I'm not sure I trust them.'"
One potential concern raised by moving COVID-19 testing into the privacy of people’s homes is that the government and public health officials could lose the ability to track new cases and outbreaks if people don’t report new infections. Mina said he’s not worried about that.
"If somebody is unwilling to just report their result, they probably were unwilling to go out and spend money and get a PCR test anyway,” Mina said. “So the point is, if we make testing more accessible, then even if we only have a fraction of those people reporting the results, that could be much more public health data than getting perfect reporting of a smaller number of PCR based tests.”
The cost of at-home testing could be a barrier for some people. Abbott is pricing its tests at $23.99 for a box of two, and two Quidel tests will go for $29.95. The single Ellume test sells for $38.99. E25Bio says it hopes to offer tests for less than $10 each.
"Currently, with the price tag, it is still a barrier for the poorest of Americans, or even just the non-wealthy," Mina said. "Very few people will want to spend that kind of money on a regular basis. So it still is creating a barrier, where the wealthy and well-off are able to get tested and know if their kid is sick with COVID, and the poor are not. And that continues to be a major travesty."
Mina said the federal government should step in to make the tests more accessible, perhaps by implementing a voucher program.
The federal government is currently testing whether free distribution of rapid antigen tests is an effective tool for reducing the spread of COVID-19. The Centers for Disease Control and Prevention and National Institutes of Health started giving away tests to residents in Pitt County, North Carolina, in March and are beginning a similar program this week in Hamilton County, Tennessee.
“Combined with efforts to increase vaccinations, this important initiative will help us understand how best to utilize these new at-home tests to reduce viral transmission rates in communities,” CDC Director Rochelle Walensky said in a press release announcing the program.
At the state level, Massachusetts officials say there are no plans for the state to buy or distribute rapid antigen tests for residents to use at home. The state has invested in making PCR testing available for free at about 35 locations around the state and is distributing self-administered PCR tests to state agencies and school districts, which are then sent to a lab for analysis.
“These tests are incredibly powerful,” said McLaren. “And if we had invested in these early on we would be in a completely different situation than we are now.”